Jamb Chemistry Past Questions For Year 2016
Question 46
N2O4(aq) \(\rightleftharpoons\) 2NO2(g) \(\bigtriangleup\)H = +ve
In the reaction above, an increase in temperature will
- A. Increase the reactant production
- B. Increase the value of the equilibrium constant
- C. Shift the equilibrium to the left
- D. Decrease the value of the equilibrium constant
Question 47
CH4(g) + CI2(g) \(\to\) CH2CI(s) + HCIg
The major factor that influences the rate of the reaction above is
- A. Concentration
- B. Catalyst
- C. Temperature
- D. Light
Question 48
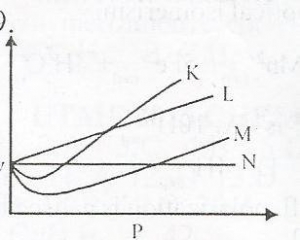
From the diagram above, an ideal gas can be represented by
- A. K
- B. M
- C. L
- D. N
Question 49
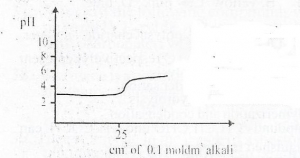
The curve depicts titration between a strong acid of pH
- A. Strong acid and strong base
- B. Strong acid and weak base
- C. Weak acid and weak base
- D. Weak acid and strong base
Question 50

The diagram represents
- A. A spontaneous reaction
- B. An exothermic reaction
- C. A non-spontaneous
- D. An endothermic reaction