Jamb Chemistry Past Questions For Year 1999
Question 6
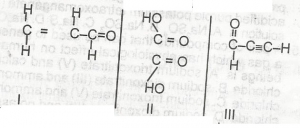
Which of the compounds above would react to take up two molecules of bromine durng bromination?
- A. l only
- B. lll only
- C. l and ll only
- D. ll and lll only
Question 7
56.00cm3 of a gas at S.T.P. weighed 0.11g. What is the vapour density of the gas?
[Molar volume of a gas at S.T.P = 22.4dm3]
- A. 11.00
- B. 22.00
- C. 33.00
- D. 44.00
Question 8
200cm3 each of 0.1M solutions of lead (II) trioxonitrate (V) and hydrochloric acid were mixed. Assuming that lead (II) chloride is completely insoluble, calculate the mass of lead (II) chloride that will be precipitated.
[Pb = 207, Cl= 35.5, N = 14, O = 16]
- A. 2.78g
- B. 5.56g
- C. 8.34g
- D. 11.12g
Question 9
A gaseous metallic chloride MClx consists of 20.22% of M by mass. The formula of the chloride is?
[M = 27, Cl = 35.5]
- A. MCl
- B. MCl2
- C. MCl3
- D. M2Cl6
Question 10
Mg(s) + 2HCl(aq) → MgCl2(aq) + H2(g)
From the equation above, the mass of magnesium required to react with 250cm3 of 0.5M HCl is
[Mg = 24]
- A. 0.3g
- B. 1.5g
- C. 2.4g
- D. 3.0g