Electrochemical Cells, Redox Series, Electrode Potentials Jamb Chemistry Past Questions
Question 1
In the electrolysis of CuSo4(g) using platinum electrode the reaction at the anode is
- A. 4H+ 4e - 2H2
- B. 4OH= 4e- + 2H2O + O2
- C. 2OH - 2e- = 2OH
- D. 2OH- + 2OH = 2H2O + O2
Question 2
In the electrolysis of brine the anode is
- A. platinum
- B. copper
- C. zinc
- D. carbon
Question 3
In an electrochemical cell, polarization is caused by
- A. Oxygen
- B. Hydrogen
- C. Tetraoxosulphate(IV) acid
- D. Chlorine
Question 4
Calculate the volume in cm3 of oxygen evolved at s.t.p when a current of 5A is passed through acidified water for 193s?
[F = 96500 C mol1. Molar volume of a gas at s.t.p = 22.4dm3]
- A. 0.056dm3
- B. 0.224dm3
- C. 224.000dm3
- D. 56.000dm3
Question 5
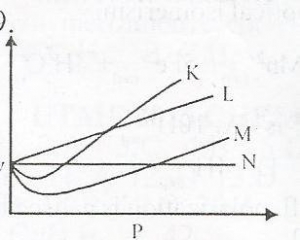
From the diagram above, an ideal gas can be represented by
- A. K
- B. M
- C. L
- D. N