Shapes Of S And P Orbitals Jamb Chemistry Past Questions
Question 1
If the relative atomic mass of an element is not a whole number, it can be deduced that the element is?
- A. Naturally radioactive
- B. abundant in nature
- C. a transition metal
- D. an isotopic mixture
Question 2
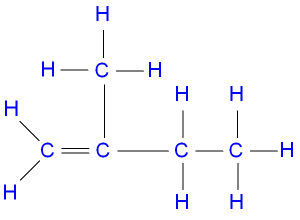
The IUPAC nomenclature of the structure above is
- A. 3-methybut-3-ene
- B. 2-methylbut-1-ene
- C. 2-ethylprop-1-ene
- D. 2-methylbut-2-ene
Question 3
An oxide XO2 has a vapour density of 32. What is the atomic mass of X?
- A. 20
- B. 32
- C. 14
- D. 12
Question 4
Elements X and Y have electronic configurations 1s\(^2\)2s\(^2\)2p\(^4\) and 1s\(^2\)2s\(^2\)2p\(^6\)3s\(^2\)3p\(^1\) respectively. When they combine, the formula of the compound formed is
- A. XY
- B. X\(_2\)Y
- C. Y\(_2\)X\(_3\)
- D. X\(_2\)Y\(_3\)
Question 5
The hybridization in the compound \(CH_3 - CH_2 - C \equiv H\) is
- A. sp\(^3\) and sp
- B. sp
- C. sp\(^2\)
- D. sp\(^3\) and sp\(^2\)